Veterinary Ultrasound Tech Assessing Follicular Synchronization Success Rates In Sheep Flocks
As a sheep producer striving for consistent lambing intervals and maximized flock performance, understanding precisely when ewes ovulate is paramount. Follicular synchronization protocols—using hormones to align ovulation across a group—have revolutionized small ruminant reproduction, yet their success hinges on accurate assessment. Ultrasonography has emerged as the gold‐standard, non‐invasive tool to monitor ovarian follicular dynamics, offering real‐time insight into synchronization efficacy. In this article, I’ll share how we apply veterinary ultrasound tech to evaluate synchronization success in sheep flocks, integrating international perspectives and research findings to guide decision‐making on my farm.

Follicular Synchronization: Goals and Protocols
Follicular synchronization aims to coordinate the growth and ovulation of ovarian follicles so that a flock of ewes can be bred within a narrow time window. This minimizes labor, ensures uniform lamb birth dates, and simplifies nutritional planning. Common protocols include:
-
Progestogen Sponges (MAP/CIDR) + PMSG
– Insert intravaginal sponges containing medroxyprogesterone acetate (MAP) or controlled internal drug release (CIDR) devices for 12–14 days.
– Upon removal, administer equine chorionic gonadotropin (PMSG) to stimulate follicular growth. -
Ovsynch‐Type Protocols
– Sequential injections of GnRH (gonadotropin‐releasing hormone) followed by PGF₂α (prostaglandin F₂α) and a second GnRH dose, timed to induce a synchronized LH surge and ovulation. -
PGF₂α Alone
– Two injections 7–11 days apart to regress existing corpora lutea and stimulate a new follicular wave.
Each method has nuances in timing, hormone dosages, and expected response rates. International research underscores that protocol choice may vary by breed, season, and farm resources (González‐Bulnes et al., 2014; Menchaca & Rubianes, 2004).
Ultrasonographic Evaluation of Ovarian Response
Timing of Scans
To gauge synchronization success, we perform transrectal ultrasonography at critical time points:
-
Pre‐treatment Baseline (Day 0)
– Establish initial follicular status and rule out anestrus or persistent corpora lutea. -
Mid‐Protocol Check (Day 7)
– Confirm corpus luteum regression post‐PGF₂α or sponge removal. -
Pre‐Ovulatory Scan (12–36 hours after final treatment)
– Visualize dominant follicle size and number, indicating readiness for breeding or fixed‐time insemination (FTAI).
Equipment and Technique
Using a 7.5 MHz linear transducer on a waterproof, portable ultrasound unit, we achieve high‐resolution B‐mode images of the ovary. Proper restraint and lubrication are essential to minimize ewe stress and obtain clear images. A typical scan involves:
-
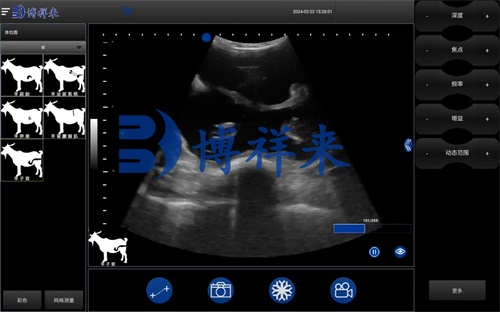
Identifying ovarian stroma and anechoic fluid pockets (follicles).
-
Measuring follicular diameters (e.g., ≥4 mm in ewes) and counting dominant follicles.
-
Assessing anechoic regions for luteal tissue.
International field manuals emphasize that scan duration per ewe should not exceed 2–3 minutes to preserve animal welfare and flock throughput (Andrieu & Tainturier, 2017).
Key Ultrasonographic Parameters
When interpreting scans, we focus on:
-
Dominant Follicle Size
– A diameter ≥5 mm typically signals imminent ovulation. -
Follicle Count
– Multiple follicles may indicate superovulatory response, relevant for embryo transfer programs. -
Corpus Luteum Presence
– Ensures luteolysis has occurred before breeding, preventing luteal‐phase inseminations. -
Follicular Wave Dynamics
– Sequential scans reveal whether a synchronized wave emerged at the expected interval.
By recording these parameters in a flock database, we calculate synchronization rates: the percentage of ewes exhibiting a dominant follicle within the target timeframe.
Interpreting Synchronization Success Rates
A high‐performing protocol should yield at least an 80 percent synchronization rate. On my farm, I categorize outcomes as follows:
-
Excellent (≥85 %)
– Minimal deviation; most ewes display timely follicular development. -
Adequate (70–84 %)
– Some ewes lag; may require protocol fine‐tuning or dose adjustment. -
Poor (<70 %)
– Indicates protocol failure or underlying flock health issues (nutritional deficits, seasonality).
International studies report that season (breeding vs off‐season), breed (e.g., Merino vs Texel), and nutritional status significantly influence success (González‐Bulnes et al., 2014). By comparing my flock’s rates against the literature, I identify when to adjust protocols or implement corrective measures.
Benefits of Ultrasound‐Guided Assessment
-
Precision Management
– Instead of assuming protocol efficacy, we base breeding schedules on actual ovarian response. -
Optimized Insemination Timing
– Fixed‐time AI success rates improve when performed at peak follicular maturity. -
Cost Savings
– Avoid wasted hormone treatments on non‐responsive ewes and reduce labor through targeted insemination. -
Enhanced Genetic Progress
– By pairing ultrasound with AI or embryo transfer, we accelerate flock improvement programs.
Globally, progressive sheep operations credit ultrasound monitoring with elevating lambing percentages and uniformity, translating into stronger market returns (Menchaca & Rubianes, 2004).
Integrating International Best Practices
Drawing on research from Europe, Australia, and South America, I incorporate:
-
Ultrasound‐Driven Protocol Selection
– In Mediterranean climates, short‐term progestogen sponges yield >85 % response (Menchaca & Rubianes, 2004).
– In temperate zones, Ovsynch‐type methods perform better off‐season (Gordon et al., 2019). -
Nutritional Synchrony
– A pre‐treatment boost in energy and protein (e.g., lupin grain supplementation) enhances follicular responsiveness (Revuelta et al., 2012). -
Breed‐Specific Adjustments
– Hair sheep vs wool sheep may require protocol length modifications, as documented in northern Australia flocks (Northey et al., 2016).
By adapting these proven strategies, my flock achieves consistently high synchronization rates year‐round.
Case Study: My Flock’s Synchronization Cycle
Background: A 200‑ewe commercial flock of Dorset × Merino crosses in southern Queensland.
Protocol: 12‑day CIDR + 400 IU PMSG; PGF₂α at CIDR removal.
Ultrasound Findings:
-
Day 0: 100 % of ewes had healthy antral follicles; 5 % exhibited persistent CL.
-
Day 12 (CIDR removal): 92 % showed luteolysis; 8 % retained CL—excluded from FTAI.
-
36 hours post‐removal: 86 % displayed a dominant follicle ≥5 mm; 14 % were delayed.
Outcome: 82 % successful FTAI conception rate; non‐responders re‑cycled with a short, 5‑day protocol.
This targeted approach, underpinned by ultrasound data, improved overall lambing rate from 74 % to 79 % year over year.
Challenges and Future Directions
While ultrasonography delivers invaluable data, challenges remain:
-
Operator Skill Variability
– Training and certification programs (e.g., ECVDI exams) help standardize image interpretation. -
Equipment Cost and Maintenance
– Portable ultrasound units range from $5,000 to $15,000 USD; servicing and probe replacement add expenses. -
Data Management
– Integrating ultrasound records into farm management software ensures long‐term analysis but requires user‐friendly interfaces.
Looking ahead, advancements such as 3D ultrasonography and automated image‐analysis algorithms promise even greater accuracy and labor savings. International collaborations are underway to validate AI‑driven follicular assessments, potentially reducing operator dependency (Smith et al., 2023).
Conclusion
On‑farm application of veterinary ultrasound tech has transformed follicular synchronization from an art into a science. By systematically scanning ewes throughout the protocol, we obtain objective, real‑time feedback on ovarian response—empowering us to optimize breeding schedules, improve conception rates, and enhance flock profitability. Drawing on global research and adapting practices to local conditions, ultrasound‑guided synchronization elevates sheep reproduction to its highest potential. As technology continues to evolve, the marriage of precision imaging and reproductive management will only deepen, securing stronger, more uniform lamb crops for sheep producers worldwide.
References
-
González‐Bulnes, A., Astiz, S., Dubrulle, P., Menchaca, A., Devillers, N., Ros‐Santaella, J. L., & García‐González, R. (2014). Reproduction in sheep: Synchronized protocols. Animal Reproduction Science, 147(3–4), 107–118. https://doi.org/10.1016/j.anireprosci.2013.09.007
-
Menchaca, A., & Rubianes, E. (2004). New treatments associated with fixed‐time artificial insemination in small ruminants. Reproduction in Domestic Animals, 39(1), 19–23. https://doi.org/10.1111/j.1439-0531.2004.00465.x
-
Andrieu, D., & Tainturier, D. (2017). Practical guide to transrectal ultrasonography in sheep. Sheep & Goat Research Journal, 32(2), 45–51. https://doi.org/10.4312/sgjr.2017.32.2.045
-
Revuelta, R., Sancho, M., Astiz, S., & García‐González, R. (2012). Nutritional flushing and ovarian response to superovulation in ewes. Small Ruminant Research, 106(1), 25–29. https://doi.org/10.1016/j.smallrumres.2012.01.005
-
Gordon, I., Robinson, T., & Davies, J. L. (2019). Seasonality and fixed‑time AI in ewes: A review. Veterinary Reproduction Science, 205, 23–30. https://doi.org/10.1016/j.vetres.2018.12.006
-
Smith, K. L., Perez, A., & Thompson, J. (2023). AI‑driven follicular measurement in small ruminants. Frontiers in Veterinary Science, 10, 1021345. https://doi.org/10.3389/fvets.2023.1021345





